Dr. Scott Guelcher and colleagues at Vanderbilt University, in conjunction with collaborators at the U.S. Army Institute of Surgical Research, and Medtronic Spine and Biologics, have recently designed a novel biomaterial that aims to stabilize fractures while remodeling to form new bone, and demonstrated proof-of-concept in a rabbit bone defect model. This project was awarded through MTEC’s Regenerative Medicine Request for Project Proposals (16-01-REGEN). For more technical detail regarding this project highlight, click here.
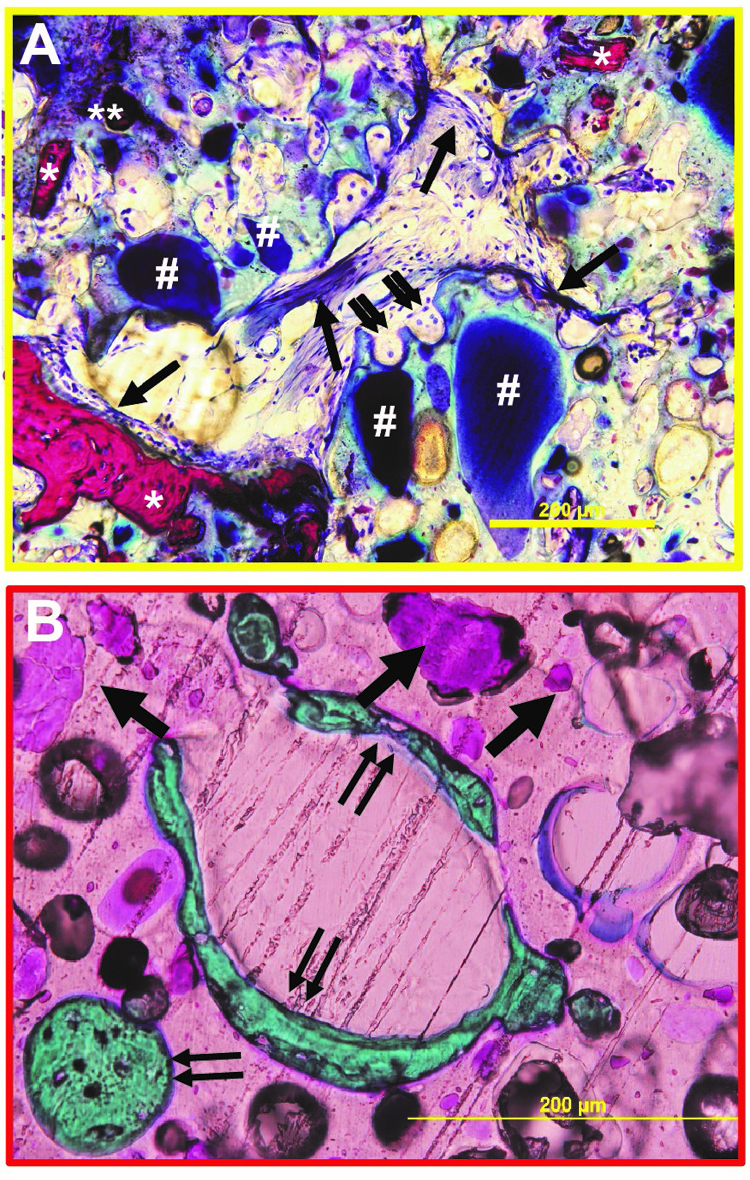
The design of biomaterials that mechanically stabilize fractures while remodeling to form new bone is an unmet challenge in bone tissue engineering. We have designed nanocrystalline hydroxyapatite (nHA)-poly(thioketal urethane) (PTKUR) bone cements that exhibit mechanical properties exceeding those of trabecular bone and undergo cell-mediated oxidative resorption. nHA-PTKUR cements were implanted in femoral plug defects in rabbits and the formation of new bone and cartilage evaluated by histology at 4, 12, and 18 months. As expected, intramembranous ossification was observed near the periphery of the cement at 4 months (Fig. A), as evidenced by osteoclast-mediated resorption of nHA-PTKUR (double arrow), osteoid (single arrow), and new bone (*). However, intramembranous ossification is limited to the host bone-cement interface and proceeds slowly. Surprisingly, endochondral ossification was also observed in the interior of the cement at 12 months (Fig. B), as evidenced by cartilage (pink, single arrow) and bone (green, double arrow) nodules. Endochondral ossification of nHA-PTKUR in response to oxidative degradation by infiltrating chondrocytes is anticipated to maintain structural support while promoting remodeling at a rate aligned with patient biology. Packaging configurations, stability-indicating methods, and kg-scale manufacturing processes have been developed to support a regulatory filing.
Madison AP McGough1, Dustin Groff1, Joseph C. Wenke2, Jeffry S. Nyman1, Cheyenne Rhodes3, Daniel A Shimko3, Craig L Duvall1, Scott A Guelcher1
1Vanderbilt University, Nashville, TN, 2US Army Institute of Surgical Research, Fort Sam Houston, TX, 3Medtronic Spine and Biologics, Memphis, TN